Wednesday, August 11, 2021
Team Knowledge Test CBSE
SOME BASIC CONCEPTS OF CHEMISTRY
PHYSICAL QUANTITIES AND SI UNITS
The 11th general conference of weights and measures in 1960 recommended the use of international system of units. Abbreviated as SI Units (after the French expression La System International de units).
FUNDAMENTAL UNITS
The SI system has seven basic units of physical quantities as follows:
Physical Quantity | Abbreviation | Name of unit | Symbol |
time | t | second | s |
mass | m | kilogram | kg |
length | l | metre | m |
temperature | T | kelvin | K |
electric current | I | ampere | A |
light intensity | Iv | candela | Cd |
amount of substance | n | mole | mol |
DERIVED UNITS
The units obtained by combination of basic units are known as derived units e.g. velocity is expressed as distance/time. Hence unit is m/s or ms–1. Some common derived units are:
Physical Quantity | Definition | SI Unit |
volume | length cube | m3 |
area | length square | m2 |
speed | distance travelled | ms–1 per unit time |
acceleration | speed changed | ms–2 per unit time |
density | mass per unit volume | kg m–3 |
pressure | force per unit area | kgm–1s–2 or Nm–2 (pressure = Pa) |
force | mass times acceleration of object | kgms–2 (Newton N) |
energy | force times distance travelled | kgm2s–2(Joule J) |
frequency | cycles per second | s–1 (hertz = Hz) |
power | energy per second | kgm2s–3 or Js–1 (Watt = W) |
electric charge | ampere times second | As (coloumb = C) |
electric potential | energy per unit | JA–1s–1 or kgm2s–3 |
difference | charge | A–1 (volt = V) |
SOME NON-SI UNITS IN COMMON USE
Quantity | Unit | Symbol | SI definition | SI Name |
Length | angstrom | Å | 10–10 m | 0.1 nanometers (nm) |
Volume | litre | L | 10–3 m3 | 1 decimeter (dm3) |
Energy | calorie | cal | kg m2s–2 | 4.184 Joule (J) |
STANDARD PREFIXES FOR EXPRESSING THE DECIMAL FRACTIONS OR MULTIPLES OF FUNDAMENTAL UNITS
Fraction | Prefix | Symbol | Multiple | Prefix | Symbol |
10–1 | deci | d | 101 | Deka | da |
10–2 | centi | c | 102 | Hecta | h |
10–3 | milli | m | 103 | kilo | k |
10–6 | micro | m | 106 | Mega | M |
10–9 | nano | n | 109 | Giga | G |
10–12 | pico | p | 1012 | Tera | T |
10–15 | femto | f | 1015 | Peta | P |
10–18 | atto | a | 1018 | Exa | E |
PRECISION AND ACCURACY
Precision : It is the closeness of various measurements for the same quantity.
Accuracy : It is the agreement of a particular value to the true value.
Example : Let the true weight of a substance be 3.00g. The measurement reported by three students are as follows
Case of A student : It is precision but no accuracy since measurements one close but not accurate.
Case of B student : Measurements are close (precision) and accurate (Accuracy)
Case of C student : Measurement are not close (no precision) and not accurate (no accuracy)
STOICHIOMETRY
Stoichiometry : It is calculation of masses or volumes of reactants and products involved in a chemically balanced reaction. Consider the formation of ammonia.
N2 (g) + 3H2 (g)  2NH3(g)
2NH3(g)
All are gases indicated by letter (g) and coefficients 3 for H2 and 2 for NH3 are called stoichiometric coefficients. The formation of ammonia can be interpreted in many ways:
- One mole of N2(g) reacts with three moles of H2(g) to give two moles of NH3(g).
- 28g of N2(g) reacts with 6g at H2(g) to give 34g of NH3(g).
- 22.4L of N2(g) reacts with 67.2L of H2(g) to give 44.8L of NH3(g)
SIGNIFICANT FIGURES
The weight 7.52 gm of a substance indicates that it is reliable to the nearest hundredth of a gram and may be expressed as 7.52 ± 0.01. It means slightest variation may occur at the second place of decimal or we can say that uncertainty is ± 0.01 g.
Now consider the weight 6.4234 g. It may correctly be expressed as 6.4234 ± 0.001 g.
In the first case the weight contains three significant figures and in the second case weight contains five significant figures.
- Significance of zero : If zero is used to locate the decimal point it is not considered as significant figure. Thus in 0.0072 there are only two significant figures whereas in 70.40, there are four significant figures since zero is after 4. Again in 0.0070 there are two significant figures, since zero after 7 is significant for it has a meaning when written in exponentials. If we compare 7.0 × 10–3 and 7 × 10–3, the first term has uncertainty of one in seventy and second has uncertainty of one in seven. The exponential term does not add to number of significant figures.
- Addition and subtraction of quantities : In this case the uncertainty in the result is equal to the sum of the uncertainties of the individual quantities.
- Multiplication and division : In this case the uncertainty in the result is equal to the sum of the percentage of individual uncertainties.
- Rounding off : The following rules are observed.
- If the digit after the last digit to be retained is less than 5, the last digit is retained as such. e.g. 1.752 = 1.75 (2 is less than 5).
- If the digit after the last digit to be retained is more than 5, the digit to be retained is increased by 1. e.g. 1.756 = 1.76 (6 is more than 5).
- If the digit after the last digit to be retained is equal to 5, the last digit is retained as such if it is even and increased by 1 if odd.
- Calculations involving addition and subtraction : In case of addition and subtraction the final result should be reported to the same number of decimal places as the number with the minimum number of decimal places .
- Calculations involving multiplication and division : In this case the final result should be reported having same number of significant digits as that of the number having least significant digits.
MATTER
Anything which occupies space, possesses mass and can be felt is called matter.
CLASSIFICATION OF MATTER
ELEMENT
Pure substance consisting of one type of particles in the form of atoms eg. Cu, Na, Fe or molecules eg. H2, O2 etc.
COMPOUND
Pure substance consisting of molecules formed by the combination of atoms of different elements eg. CO2, H2O etc.
MIXTURES
Mixtures are substances made of two or more elements or compounds in any proportion. They may be homogeneous or heterogeneous.
SEPARATION OF MIXTURES
Mixtures can be separated into constituents by following methods:
- Filtration can separate those mixtures whose one component is soluble in a particular solvent and other is not.
- Distillation can be used to separate constituents of mixtures having different boiling points.
- Extraction dissolves one out of several components of mixture.
- Crystallisation is a process of separating solids having different solubilities in a particular solvent.
- Sublimation separates volatile solids which sublime on heating from non-volatile solids.
- Chromatography is the technique of separating constituents of a mixture which utilises the property of difference of adsorption on a particular adsorbent.
- Gravity separation separates constituents having different densities.
- Magnetic separation can separate magnetic components from non magnetic ones.
PHYSICAL AND CHEMICAL CHANGES
A change which does not affect chemical composition and molecular structure is a physical change and the one that involves alteration of chemical composition and molecular structure is a chemical change.
- Chemical Combination is reaction between two or more elements or compounds to form a single substance.
H2 + I2  2HI
2HI
- Displacement means replacement of one element of compound by another.
- Decomposition involves splitting of a compound to form two or more substances.
- Combustion is a complete and fast oxidation of a substance.
- Neutralisation is the reaction between acid and base to form a salt.
- Polymerisation is the combination of molecules of same or different substances to form a single molecule called polymer
- Photochemical changes occur in presence of visible or ultraviolet light.
- Double decomposition or metathesis is the exchange of oppositely charged ion on mixing two salt solutions.
- Hydrolysis involves reaction of salts with water to form acidic or basic solutions.
LAWS OF CHEMICAL COMBINATIONS
- Law of conservation of mass : This law was given by French chemist A. Lavoisier (1774) which states that "during any physical or chemical change, the total mass of products is equal to the total mass of reactants". It is also called law of indistinctibility. It does not hold good for nuclear reaction.
- Law of definite proportions : This law was given by Proust (1799) and states that "a chemical compound always contains some elements combined together in same proportion by mass". For example different samples of pure CO2 always have carbon and oxygen in 3 : 8 ratio by mass.
- Law of multiple proportions : This law was given by John Dalton (1803) and states that "when two elements combine to form two or more compounds, the different mass of one of the elements and the fixed mass of the one with which it combines always form a whole number ratio". This law explains the concept of formation of more than one compound by two elements.
- Law of reciprocal proportions : This was given by Richter (1792) and states that "when two elements combine separately with a fixed mass of third, the ratio of masses in which they do so is same or whole number multiple of the ratio in which they combine with each other." This law is also called law of equivalent proportions and is helpful in determining equivalent weights.
- Gay Lussac's law of combining volumes : This law states that when gases react with each other, their volumes bear a simple whole no. ratio to one another and to volume of products (if gases) and similar conditions of pressure and temperature.
- Dalton's atomic theory :
- Proposed by John Dalton in 1808. Main points are :
- Matter is made up, by indivisible particles called atoms
- Atoms of same elements are identical in physical and chemical properties.
- Atoms of different substances are different in every respect
- Atoms always combine in whole numbers to form compounds
- Atoms of resultant compounds possess similar properties
Drawbacks of Dalton's theory :
- Does not explain structure of atom.
- Fails to explain binding forces between atoms in compounds.
- Does not explain Gay Lussac's law.
- Does not differentiate between atom and molecule.
- Avogadro's law :
It states that "equal volumes of all gases, under similar conditions of temperature and pressure contain equal number of molecules". Applications are:
- Deducing atomicity of elementary gases
- Deriving relationship between molecular mass and vapour density
- Deriving formula of substances
- Determining molecular wt. of a gas
- Deducing the gram molecular volume.
ATOM
Atom is the smallest particle of element which might not be able to exist independently.
MOLECULE
Molecule is the smallest particle of the substance which can exist independently. It can be subdivided as
- Homoatomic molecules are molecules of same element and can be further divided as monoatomic, diatomic and polyatomic molecules depending upon number of atoms. eg: He, O2, P4 etc.
- Heteroatomic molecules are molecules of compound. They can be diatomic and polyatomic. eg: H2O, PCl5, H2SO4, NO etc.
ATOMIC MASS UNIT (A.M.U.)
It is the unit of representing atomic masses. 1 a.m.u. = th the mass of C-12.
th the mass of C-12.
MOLE
It is a unit which represents 6.023 × 1023 particles. The number 6.023 × 1023 is called Avogadro's number and is represented by N0 or NA. Avogadro's number of gas molecules occupy a volume of 22400 cm3 at N.T.P. Number of molecules in 1 cm3 of gas at NTP is Loschmidt N0. With value 2.688 × 1019.
ATOMIC MASS
"It is the number of times the atom of the element is heavier than H atom" was the first proposed definition. Later on oxygen was preferred as standard. In 1961 C-12 was chosen as standard and thus "the number of times the atom of an element is heavier than 12th part of C-12 is called atomic mass of the element.
Atomic mass = 
AVERAGE ATOMIC MASS
It is the mass of each isotope determined separately and then combined in ratio of their occurrence. Suppose a and b are two isotopes of an element with their occurence ratio p : q then
Average atomic mass = 
DETERMINATION OF ATOMIC MASS
- Dulong and petit's rule : It is based on experimental facts. "At ordinary temperature, product of atomic mass and specific heat for solid elements is approximately 6.4 and this product is known as atomic heat of the element"
Atomic mass × specific heat = 6.4
The law is valid for solid elements except Be, B, Si and C.
Correct At. mass = Eq. mass × valency
- Specific heat method : This method is for gases.
, where Cp = specific heat at constant pressure and Cv = specific heat at constant volume. the ratio g is a constant = 1.66 for monoatomic, 1.40 for diatomic, 1.33 for triatomic gas and atomic mass of gaseous element
=.
- Chloride formation method : This method converts the element (whose mass is to be determined) into volatile chloride whose vapour density is found by Victor Mayer method.
Molecular mass = 2 × V.D.
- Vapour density method is suitable for elements having volatile chlorides.
Atomic mass = Eq. mass of metal × valency.
- Mitscherlich's law of isomorphism : It states that isomorphous substances have similar chemical constitution. Isomorphous substances form crystals of same shape and valencies of elements forming isomorphous salts are also same. eg: ZnSO4. 7H2O, MgSO4.7H2O and FeSO4.7H2O are isomorphous.
GRAM ATOMIC MASS (GAM)
Is the mass of an atom expressed in gms.
No. of Gm-atoms of element = 
MOLECULAR MASS :
It is the average relative mass of the molecule as compared with mass of C-12 atom.
Molecular mass = 
CALCULATION OF MOLECULAR MASS :
- Graham's law of diffusion : It states that rate of diffusion of two gases is inversely proportional to the square root of ratio of their molecular weights.
- Victor meyer method : This method can determine the molecular mass as
Molecular mass = × 22400
× 22400
where W is the mass of liquid in gm. occupying a volume V ml at STP.
- Vapour density method : Vapour density is the ratio of volume of a gas to the mass of same volume of hydrogen under identical conditions.
or 
Thus molecular mass = 2 × V.D.
- Colligative properties method : This method can be helpful in determining molecular mass as
elevation in boiling point 
Where  Tb is elevation in b.p., Kb is molal elevation constant w is wt. of solute W is wt. of solvent
Tb is elevation in b.p., Kb is molal elevation constant w is wt. of solute W is wt. of solvent
Depression in freezing point 
GRAM MOLECULAR MASS OR MOLAR MASS :
That amount of substance whose mass in grams is equal to its molecular mass or the equivalently molecular mass of a substance expressed in grams is called gram molecular mass. Gram molecular mass is also called one gram molecule. thus
No. of gm molecules = 
EQUIVALENT MASS :
It is the number of parts by weight of the substance that combines or displaces, directly or indirectly, 1.008 parts by mass of hydrogen or 8 parts by mass of oxygen or 35.5 parts by mass of chlorine. It can be calculated as-
- Equivalent mass for elements =
- Equivalent mass for acids =
- Equivalent mass for bases =
- Equivalent mass for salts =
- Equivalent mass for oxidising agents =
- Equivalent mass for reducing agents =
- Equivalent weight of radicals =
FORMULA MASS :
It is obtained by adding atomic masses of various atoms present in the formula and this term replaces molecular mass in ionic compounds.
ACIDITY :
It is the number of OH– ions that can be displaced from one molecule of a substance.
BASICITY :
It is the number of H+ ions that can be displaced from one molecule of a substance.
GRAM EQUIVALENT MASS (GEM) :
It is the mass of a substance expressed in grams or equivalently the quantity of substance whose mass in grams is equal to its equivalent mass is called one gram equivalent or gram equivalent mass.
No. of gm equivalents =  .
.
METHODS OF DETERMINING EQUIVALENT MASSES :
- Hydrogen displacement method : It is for metals which can displace H2 from acids.
Equivalent mass of metal
= 
- Metal displacement method : It utilises the fact that one GEM of a more electropositive metal displaces one GEM of a less electropositive metal from its salt
.
- Conversion method : When one compound of a metal is converted to another compound of similar metal then
where E is the eqv. mass of the metal.
- Electrolytic method :
It states that the quantity of substance that reacts at electrode when Faraday of electricity is passed is equal to its GEM.
GEM = Electrochemical equivalent × 96500
and ratio of weights deposited by equal amount of electricity is in ratio of their equivalent masses.
- Oxide method :
Equivalent mass of metal = 

- Double decomposition :
- Neutralisation method for acids and bases :
Equivalent mass of acid (base)
= 
- Silver salt is method commonly used for organic acids.
Eqv. mass of acid = 
Mol. mass of acid = Eqv. mass of acid × Basicity
- Platinichloride method for bases :
Eqv. mass of base
=
Mol. mass of base = Eqv. mass of base × Acidity
- Chloride method :
Eqv. mass of metal = 
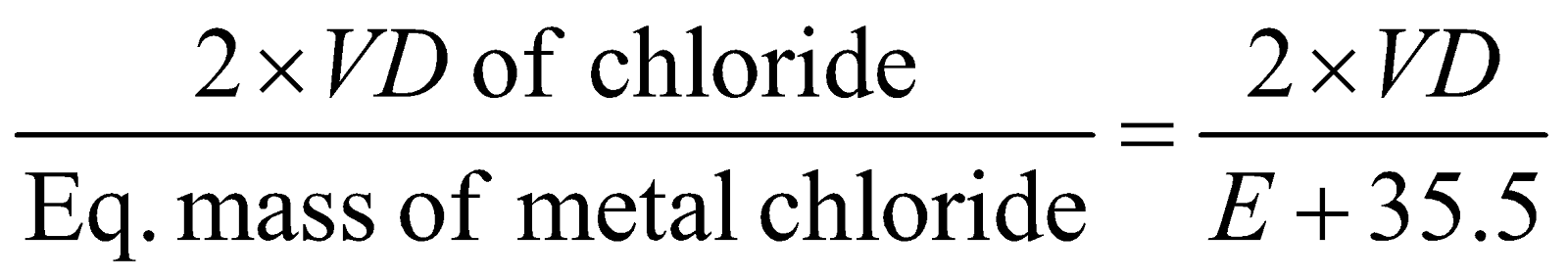
- Volatile chloride method :
Valency of metal
= 
CHEMICAL EQUATION :
It is the equation representing chemical change in terms of formula of reactants and products.
- An equation which has not been equalised in terms of number of atoms of reactants and products is called a skeleton equation.
- An equation having equal number of atoms of various kinds on both sides is a balanced equation.
EMPIRICAL FORMULA :
It is the simplest formula of a compound giving simplest whole number ratio of atoms present in one molecule. e.g. CH is empirical formula of benzene.
MOLECULAR FORMULA :
It is the actual formula of a compound showing the total number of atoms of constituent elements e.g. C6H6 is molecular formula of benzene.
Molecular formula = n × empirical formula, where n is simple whole number.
SOLUTION :
It is a homogenous mixture of two or more substances. The component of solution having larger proportion is solvent and others are solute.
MOLE FRACTION :
It is the ratio of moles of a constituent to the total number of moles in a solution.
Let A be solute & B is solvent then mole fraction of solute (xA)
= , where n is the number of moles.
, where n is the number of moles.
Mole fraction of solution 
MASS PERCENTAGE :
It is the number of parts by mass of solute per hundred parts by mass of solution. If WA is mass of solute and WB the mass of solvent, then
Mass percentage of A = . 
VOLUME PERCENTAGE :
It is the number of parts by volume of solute per hundred parts by volume of solution. If VA is volume of solute and VB is the volume of solvent then
Volume percentage of A = 
PARTS PER MILLION (PPM) :
It is the mass of solute present in one million parts by mass of solution.
NORMALITY :
It is the number of gram equivalents of a solute present in one litre of solution.
Normality depends on temperature. Also if strength is given in normalities, N1 of A & N2 of B
Then N1V1 = N2V2.
MOLARITY :
It is the number of moles of solute present in one litre of solution.
and millimoles = M × V(in ml).
Molarity and mass percentage have the relation M
If a solution of molarity M1 and volume V1 adds up with a solvent to a final volume V2, then molarity M2 is given by
If two different solutions (M1, V1) and (M2, V2) are mixed then molarity of resulting solution is
M = 
Also, Molarity × GMM of solute = Normality × GEM of solute
MOLALITY :
It is the number of moles of solute in 1 kg of solvent.
Molality (m) = 

Molality is independent of temperature.
FORMALITY (F) :
It is the number of gram formula mass of ionic solute dissolved in 1 litre of solution.
Formality = 
LIMITING REAGENT :
It is the reactant which is completely consumed during the reaction.
Tags :
some basic concepts of chemistry
class 1 notes
cbse notes
life processes class 10 notes
class 10 science notes
life processes class 10
heredity and evolution class 10 notes
class 9 science notes
electricity class 10 notes
power sharing class 10 notes
class 11 physics notes
class 11 chemistry notes
resources and development class 10 notes
our environment class 10 notes
maths class 10 notes
manufacturing industries class 10
carbon and its compounds class 10 notes
heredity and evolution class 10
class 10 history chapter 1 notes
metals and non metals class 10
federalism class 10 notes
development class 10 notes
political parties class 10 notes
manufacturing industries class 10 notes
agriculture class 10 notes
chemical reaction and equation class 10 notes
political parties class 10
physics notes for class 10
p block elements class 12
class 11 chemistry chapter 1
money and credit class 10 notes
light reflection and refraction class 10 notes
class 12 chemistry notes
electrochemistry class 12 notes
class 11 chemistry chapter 1 notes
our environment class 10
matter in our surroundings class 9 notes
class 10 geography chapter 1 notes
class 11 biology notes
acid bases and salts class 10 notes
class 12 physics notes
light class 10 notes
some basic concepts of chemistry class 11
ray optics class 12 notes
metals and non metals class 10 notes
haloalkanes and haloarenes class 12 notes
class 10 chemistry chapter 1 notes
class 10 biology chapter 1 notes
class 12 biology notes
p block elements class 12 notes
chemical kinetics class 12 notes
class 10 science chapter 1 notes
class 10 economics chapter 1 notes
class 12 physics chapter 1 notes
class 12 english notes
light reflection and refraction class 10
refraction of light class 10
history class 10 chapter 1 notes
basic concepts of chemistry
the french revolution class 9 notes
class 9 history chapter 1 notes
wave optics class 12 notes
light class 10
class 11 chemistry chapter 2 notes
class 11 physics chapter 2 notes
electric charges and fields class 12 notes
class 10 civics chapter 1 notes
current electricity class 12 notes
some basic concepts of chemistry class 11 notes
some basic concepts of chemistry notes
solutions class 12 notes
surface chemistry class 12 notes
class 10 social science notes
class 8 science chapter 1 notes
lifelines of national economy class 10 notes
class 11 biology chapter 1 notes
class 10 notes
class 12 chemistry chapter 1 notes
class 11 maths notes
class 10 chemistry notes
physics wallah notes class 12
reproductive health class 12 notes
solid state class 12 notes
coordination compounds class 12 notes
physics class 11 chapter 1 notes
class 12 physics handwritten notes
some basic concepts of chemistry notes pdf
class 10 sst notes
plus one english notes
english notes class 10
human eye and colourful world class 10 notes
magnetism and matter class 12 notes
class 10 science chapter 6 notes
class 10 science notes chapter 1
class 9 science chapter 1 notes
class 7 science chapter 1 notes
arithmetic progression class 10 notes
human health and disease class 12 notes
class 10 physics chapter 1 notes
matter in our surroundings notes
some basic concepts of chemistry class 11 notes
some basic concepts of chemistry notes pdf
some basic concepts of chemistry class 11 handwritten notes
some basic concepts of chemistry class 11 ncert pdf
some basic concepts of chemistry questions
some basic concepts of chemistry class 11 pdf
some basic concepts of chemistry ncert
some basic concepts of chemistry class 11 ncert solutions
some basic concepts of chemistry all formulas
some basic concepts of chemistry all formulas pdf
some basic concepts of chemistry answers
some basic concepts of chemistry assertion and reason
some basic concepts of chemistry all topics
some basic concepts of chemistry assignment
some basic concepts of chemistry askiitians
some basic concepts of chemistry arvind arora
answers of some basic concepts of chemistry
some basic concepts of chemistry best notes
some basic concepts of chemistry byju's
some basic concepts of chemistry book pdf
some basic concepts of chemistry by physics wallah
some basic concepts of chemistry by arvind arora
some basic concepts of chemistry by unacademy
some basic concepts of chemistry by vedantu
some basic concepts of chemistry book
some basic concepts of chemistry class 11 notes pdf
some basic concepts of chemistry class 11 notes pdf download
some basic concepts of chemistry class 11 notes study rankers
ch 1 some basic concepts of chemistry
some basic concepts of chemistry dpp
some basic concepts of chemistry deleted portion
some basic concepts of chemistry definition
some basic concepts of chemistry exercise
some basic concepts of chemistry extra questions
some basic concepts of chemistry exemplar
some basic concepts of chemistry entrance questions and answers pdf
some basic concepts of chemistry explanation
some basic concepts of chemistry exam fear
some basic concepts of chemistry edumantra
some basic concepts of chemistry exercise pdf
some basic concepts of chemistry formula sheet
some basic concepts of chemistry formulas
some basic concepts of chemistry full chapter
some basic concepts of chemistry flow chart
some basic concepts of chemistry for neet
some basic concepts of chemistry for jee
some basic concepts of chemistry formulas for neet
some basic concepts of chemistry formula sheet for neet
some basic concepts of chemistry gneet
some basic concepts of chemistry gravity circle
some basic concepts of chemistry grade 11
some basic concepts of chemistry handwritten notes
some basic concepts of chemistry handwritten notes for neet
some basic concepts of chemistry hard questions
some basic concepts of chemistry hindi
some basic concepts of chemistry hsslive
some basic concepts of chemistry important questions
some basic concepts of chemistry in hindi pdf
some basic concepts of chemistry iit jee notes pdf
some basic concepts of chemistry important topics
some basic concepts of chemistry important questions for neet
some basic concepts of chemistry important topics for neet
some basic concepts of chemistry important notes
some basic concepts of chemistry important formulas
some basic concepts of chemistry jee questions
some basic concepts of chemistry jee
some basic concepts of chemistry jee mains previous year questions
some basic concepts of chemistry jee notes
some basic concepts of chemistry jee advanced questions
some basic concepts of chemistry jee mains
some basic concepts of chemistry jee main questions
some basic concepts of chemistry jee mock test
some basic concepts of chemistry khan academy
some basic concepts of chemistry kota notes
some basic concepts of chemistry keam questions
some basic concepts of chemistry kcet questions
some basic concepts of chemistry ke notes
some basic concepts of chemistry kcet questions pdf
some basic concepts of chemistry kcet notes
some basic concepts of chemistry kcet
some basic concepts of chemistry learn cbse
some basic concepts of chemistry laws
some basic concepts of chemistry learnohub
some basic concepts of chemistry lesson pdf
some basic concepts of chemistry lesson plan
some basic concepts of chemistry mcq
some basic concepts of chemistry meaning in hindi
some basic concepts of chemistry mcq for neet
some basic concepts of chemistry mcq for neet pdf download
some basic concepts of chemistry mind map
some basic concepts of chemistry mcq pdf
some basic concepts of chemistry mock test
some basic concepts of chemistry mole concept
some basic concepts of chemistry notes
some basic concepts of chemistry neet questions
some basic concepts of chemistry neet
some basic concepts of chemistry ncert pdf
some basic concepts of chemistry notes for neet
some basic concepts of chemistry ncert solutions
some basic concepts of chemistry chapter
ncert solutions of some basic concepts of chemistry
some basic concepts of chemistry objective questions
some basic concepts of chemistry one shot
some basic concepts of chemistry online test
some basic concepts of chemistry one mark questions
some basic concepts of chemistry of class 11
some basic concepts of chemistry one page notes
some basic concepts of chemistry objective questions pdf
1. some basic concepts of chemistry
some basic concepts of chemistry pdf
some basic concepts of chemistry pdf notes
some basic concepts of chemistry physics wallah
some basic concepts of chemistry previous year neet questions
some basic concepts of chemistry ppt
some basic concepts of chemistry previous year jee questions
some basic concepts of chemistry practice questions
some basic concepts of chemistry pdf download
some basic concepts of chemistry questions and answers
some basic concepts of chemistry questions and answers pdf
some basic concepts of chemistry question bank
some basic concepts of chemistry questions for neet
some basic concepts of chemistry quiz
some basic concepts of chemistry questions for jee
some basic concepts of chemistry question bank pdf
some basic concepts of chemistry revision notes
some basic concepts of chemistry revision
some basic concepts of chemistry problems
some basic concepts of chemistry solutions
some basic concepts of chemistry slideshare
some basic concepts of chemistry short notes
some basic concepts of chemistry short notes pdf
some basic concepts of chemistry short notes for neet
some basic concepts of chemistry summary
some basic concepts of chemistry solutions pdf
some basic concepts of chemistry study adda
some basic concepts of chemistry test
some basic concepts of chemistry topics
some basic concepts of chemistry test paper
some basic concepts of chemistry tricks
some basic concepts of chemistry toppr
some basic concepts of chemistry textbook pdf
some basic concepts of chemistry topic wise questions
some basic concepts of chemistry tiwari
some basic concepts of chemistry unacademy
some basic concepts of chemistry unacademy jee
some basic concepts of chemistry vedantu
some basic concepts of chemistry video
some basic concepts of chemistry vedantu neet
some basic concepts of chemistry vedantu jee
some basic concepts of chemistry viva questions
some basic concepts of chemistry weightage in neet
some basic concepts of chemistry worksheet
some basic concepts of chemistry weightage in jee mains
some basic concepts of chemistry wikipedia
some basic concepts of chemistry weightage
some basic concepts of chemistry worksheet pdf
some basic concepts of chemistry youtube
some basic concepts of chemistry 11th
some basic concepts of chemistry 1 mark questions
some basic concepts of chemistry 11th class
some basic concepts of chemistry 11th pdf
some basic concepts of chemistry 1st puc
chapter 1 some basic concepts of chemistry
unit 1 some basic concepts of chemistry
some basic concepts of chemistry solved exercise
geography class 10 chapter 1 notes
ncert solutions class 11 chemistry chapter 1
class 11 biology chapters
class 12 biology chapters
history chapter 1 class 10 notes
molecular basis of inheritance class 12 notes
d and f block elements class 12
human eye and colourful world class 10
reproductive health class 12
class 9 economics chapter 1 notes
class 10 biology notes
class 10 science chapter 2 notes
class 10 economics chapter 2 notes
metal and non metals class 10
class 11 chemistry chapter 3 notes
ncert notes for class 10 science
plus one malayalam chapter notes
physical education class 12 notes
electricity chapter class 10 notes
class 9 science chapter 2 notes
my cbse guide class 12
biomolecules class 12 notes
class 9 chemistry chapter 1 notes
globalisation and the indian economy class 10 notes
class 9th science notes
class 9 geography chapter 1 notes
lifelines of national economy class 10
class 12 history notes
basic concepts of chemistry class 11
social science class 9 notes
class 12 political science notes
economics class 10 chapter 1 notes
ch 6 science class 10 notes
class 12 biology chapter 1 notes
chemistry class 10 chapter 1 notes
metal and non metal class 10 notes
class 10 science notes in hindi
manufacturing industries class 10 solutions
the human eye and the colourful world class 10 notes
class 10 chemistry chapter 2 notes
class 9 biology chapter 1 notes
real numbers class 10 notes
chapter 1 science class 9 notes
class 11 chemistry chapter 1 pdf
organism and population class 12 notes
class 10 history chapter 2 notes
class 6 science chapter 1 notes
electrostatics class 12 notes
class 10 geography chapter 4 notes
class 9 civics chapter 1 notes
globalisation class 10 notes
class 12 maths notes
life processes class 10 ncert notes
class 10 economics chapter 2
plus one chemistry notes
class 11 physics chapter 3 notes
ncert class 9 science notes
plus one physics notes
d and f block elements class 12 notes
class 9 physics chapter 1 notes
light chapter of class 10 notes
class 10 science chapters
amines class 12 notes
alternating current class 12 notes
class 11 business studies chapter 1 notes
notes of history class 10 chapter 1
solid state chemistry class 12 notes
class 10 maths chapter 1 notes
class 10 civics chapter 2 notes
biology class 10 chapter 1 notes
class 12 chemistry chapter 2 notes
ray optics class 12 ncert solutions
business studies class 12 notes
reflection of light class 10 notes
ch 1 science class 10 notes
class 9 science chapter 5 notes
biotechnology principles and processes class 12 notes
continuity and differentiability class 12 notes
class 11 business studies chapter 1
statistics class 10 notes
some basic concepts of chemistry class 11 ncert solutions
chemistry class 12 chapter 1 notes
class 12 biology chapter 2 notes
physics notes for class 9th chapter 1
class 10 science chapter 10 notes
civics class 10 chapter 1 notes
science class 10 chapter 1 notes
class 10 science chapter 8 notes
class 8 science notes chapter 1
matrices class 12 notes
class 8 geography chapter 1 notes
class 6 geography chapter 1 notes
class 9 science chapter 3 notes
human eye class 10 notes
class 11 chemistry chapter 4 notes
class 11 biology chapter 2 notes
refraction of light class 10 notes
class 8 history chapter 1 notes
class 12 notes
class 9th maths notes
geography class 10 chapter 1 notes
ncert solutions class 11 chemistry chapter 1
class 11 biology chapters
class 12 biology chapters
history chapter 1 class 10 notes
molecular basis of inheritance class 12 notes
d and f block elements class 12
human eye and colourful world class 10
reproductive health class 12
class 9 economics chapter 1 notes
class 10 biology notes
class 10 science chapter 2 notes
class 10 economics chapter 2 notes
metal and non metals class 10
class 11 chemistry chapter 3 notes
ncert notes for class 10 science
plus one malayalam chapter notes
physical education class 12 notes
electricity chapter class 10 notes
class 9 science chapter 2 notes
my cbse guide class 12
biomolecules class 12 notes
class 9 chemistry chapter 1 notes
globalisation and the indian economy class 10 notes
class 9th science notes
class 9 geography chapter 1 notes
lifelines of national economy class 10
class 12 history notes
basic concepts of chemistry class 11
social science class 9 notes
class 12 political science notes
economics class 10 chapter 1 notes
ch 6 science class 10 notes
class 12 biology chapter 1 notes
chemistry class 10 chapter 1 notes
metal and non metal class 10 notes
class 10 science notes in hindi
manufacturing industries class 10 solutions
the human eye and the colourful world class 10 notes
class 10 chemistry chapter 2 notes
class 9 biology chapter 1 notes
real numbers class 10 notes
chapter 1 science class 9 notes
class 11 chemistry chapter 1 pdf
organism and population class 12 notes
class 10 history chapter 2 notes
class 6 science chapter 1 notes
electrostatics class 12 notes
class 10 geography chapter 4 notes
class 9 civics chapter 1 notes
globalisation class 10 notes
class 12 maths notes
life processes class 10 ncert notes
class 10 economics chapter 2
plus one chemistry notes
class 11 physics chapter 3 notes
ncert class 9 science notes
plus one physics notes
d and f block elements class 12 notes
class 9 physics chapter 1 notes
light chapter of class 10 notes
class 10 science chapters
amines class 12 notes
alternating current class 12 notes
class 11 business studies chapter 1 notes
notes of history class 10 chapter 1
solid state chemistry class 12 notes
class 10 maths chapter 1 notes
class 10 civics chapter 2 notes
biology class 10 chapter 1 notes
class 12 chemistry chapter 2 notes
ray optics class 12 ncert solutions
business studies class 12 notes
reflection of light class 10 notes
ch 1 science class 10 notes
class 9 science chapter 5 notes
biotechnology principles and processes class 12 notes
continuity and differentiability class 12 notes
class 11 business studies chapter 1
statistics class 10 notes
some basic concepts of chemistry class 11 ncert solutions
chemistry class 12 chapter 1 notes
class 12 biology chapter 2 notes
physics notes for class 9th chapter 1
class 10 science chapter 10 notes
civics class 10 chapter 1 notes
science class 10 chapter 1 notes
class 10 science chapter 8 notes
class 8 science notes chapter 1
matrices class 12 notes
class 8 geography chapter 1 notes
class 6 geography chapter 1 notes
class 9 science chapter 3 notes
human eye class 10 notes
class 11 chemistry chapter 4 notes
class 11 biology chapter 2 notes
refraction of light class 10 notes
class 8 history chapter 1 notes
class 12 notes
class 9th maths notes
some basic concepts of chemistry
class 1 notes
cbse notes
life processes class 10 notes
class 10 science notes
life processes class 10
heredity and evolution class 10 notes
class 9 science notes
electricity class 10 notes
power sharing class 10 notes
class 11 physics notes
class 11 chemistry notes
resources and development class 10 notes
our environment class 10 notes
maths class 10 notes
manufacturing industries class 10
carbon and its compounds class 10 notes
heredity and evolution class 10
class 10 history chapter 1 notes
metals and non metals class 10
federalism class 10 notes
development class 10 notes
political parties class 10 notes
manufacturing industries class 10 notes
agriculture class 10 notes
chemical reaction and equation class 10 notes
political parties class 10
physics notes for class 10
p block elements class 12
class 11 chemistry chapter 1
money and credit class 10 notes
light reflection and refraction class 10 notes
class 12 chemistry notes
electrochemistry class 12 notes
class 11 chemistry chapter 1 notes
our environment class 10
matter in our surroundings class 9 notes
class 10 geography chapter 1 notes
class 11 biology notes
acid bases and salts class 10 notes
class 12 physics notes
light class 10 notes
some basic concepts of chemistry class 11
ray optics class 12 notes
metals and non metals class 10 notes
haloalkanes and haloarenes class 12 notes
class 10 chemistry chapter 1 notes
class 10 biology chapter 1 notes
class 12 biology notes
p block elements class 12 notes
chemical kinetics class 12 notes
class 10 science chapter 1 notes
class 10 economics chapter 1 notes
class 12 physics chapter 1 notes
class 12 english notes
light reflection and refraction class 10
refraction of light class 10
history class 10 chapter 1 notes
basic concepts of chemistry
the french revolution class 9 notes
class 9 history chapter 1 notes
wave optics class 12 notes
light class 10
class 11 chemistry chapter 2 notes
class 11 physics chapter 2 notes
electric charges and fields class 12 notes
class 10 civics chapter 1 notes
current electricity class 12 notes
some basic concepts of chemistry class 11 notes
some basic concepts of chemistry notes
solutions class 12 notes
surface chemistry class 12 notes
class 10 social science notes
class 8 science chapter 1 notes
lifelines of national economy class 10 notes
class 11 biology chapter 1 notes
class 10 notes
class 12 chemistry chapter 1 notes
class 11 maths notes
class 10 chemistry notes
physics wallah notes class 12
reproductive health class 12 notes
solid state class 12 notes
coordination compounds class 12 notes
physics class 11 chapter 1 notes
class 12 physics handwritten notes
some basic concepts of chemistry notes pdf
class 10 sst notes
plus one english notes
english notes class 10
human eye and colourful world class 10 notes
magnetism and matter class 12 notes
class 10 science chapter 6 notes
class 10 science notes chapter 1
class 9 science chapter 1 notes
class 7 science chapter 1 notes
arithmetic progression class 10 notes
human health and disease class 12 notes
class 10 physics chapter 1 notes
matter in our surroundings notes
plus one hindi notes
alcohols phenols and ethers class 12 notes
class 11 chemistry chapter 1 important questions with answers
class 10 light reflection and refraction
some basic concepts of chemistry pdf
physics class 10 chapter 1 notes
atoms class 12 notes
class 10 science chapter 3 notes
nuclei class 12 notes
chemical reaction and equation notes
economics chapter 1 class 10 notes
triangles class 10 notes
notes of biology class 11 chapter 1
physical education class 11 chapter 1 notes
age of industrialisation class 10
class 7 geography chapter 1 notes
class 11 economics chapter 1
ch 1 history class 10 notes
class 9 science chapter 8 notes
metal and non metals class 10 notes
geography chapter 1 class 10 notes
bricks beads and bones notes
organic chemistry chapters of class 12
business studies class 11 chapter 1 notes
chemistry in everyday life class 12
physics guide for class 12
class 5 science chapter 1 notes
relations and functions class 12 notes
tissue chapter class 9 notes
water resources class 10 notes
class 10 economics chapter 3 notes
some basic concepts of chemistry class 11 pdf
class 12 physics chapter 2 notes
cbse class 7 science notes
class 11 biology notes chapter 1
electrostatic potential and capacitance class 12 notes
class 10 geography chapter 6 notes
economics class 12 notes
human eye and colourful world notes
science notes class 8
chapter 1 chemistry class 12
principles of inheritance and variation class 12
class 11 accounts chapter 1 notes
vedantu notes
study material for class 10
heredity class 10 notes
class 9 science chapter 4 notes
plus one zoology notes
biodiversity and conservation class 12 notes
class 10 manufacturing industries notes
some basic concepts of chemistry class 11 questions and answers pdf
plus one botany notes
class 7 science notes chapter 1
heredity and evolution class 10 ncert solutions
edumantra class 10 science notes
class 9 maths chapter 1 notes
8th standard english notes of lesson 1
thermodynamics class 11 chemistry notes
class 10 science chapter 5 notes
forest and wildlife resources class 10 notes
chapter 2 physics class 12
chapter 2 science class 9 notes
class 8 maths chapter 1 notes
class 10 science life process notes
science class 8 chapter 1 notes
current electricity class 12 ncert solutions
notes of business studies class 11 chapter 1
biotechnology class 12 notes
moving charges and magnetism class 12 notes
properties of light class 10
science chapter 2 class 9 notes
class 10 science chapter 13 notes
class 11 microeconomics chapter 1 notes
9th class math notes chapter 1
class 10 science ch 6 notes
class 11 economics chapter 1 notes
class 6 civics chapter 1 notes
number system class 9 notes
class 8 civics chapter 1 notes
p block elements class 12 ncert solutions
class 9 science notes in hindi
management of natural resources class 10 notes
class 11 english guide
federalism notes class 10
plus one maths chapters
class 12 physics chapter 1 electric charges and fields notes
class 10 science chapter 12 notes
coordination compounds class 12 ncert solutions
quadratic equation class 10 notes
chapter 1 physics class 12 notes
class 10 history notes
notes of agriculture class 10
class 10 light reflection and refraction notes
probability class 10 notes
p block elements class 12 ncert
class 10 science biology chapters
our environment class 10 ncert solutions
class 6 science chapter 5 notes
class 10 biology chapters
class 12 biology chapter 3 notes
class 10 science ch 1 notes
class 10 history chapter 3 notes
geography class 10 notes
class 7 history chapter 1 notes
history chapter 2 class 10 notes
cbse class 10 science notes
chapter 1 geography class 10 notes
biotechnology and its applications class 12 notes
class 11 physics chapter 4 notes
civics chapter 1 class 10
economics class 11 chapter 1 notes
class 10 science chapter 4 notes
chapter 1 physics class 11
notes of class 11 chemistry chapter 1
class 10 science light reflection and refraction notes
electromagnetic waves class 12 notes
human health and disease class 12 ncert
class 9 science handwritten notes
planning in sports class 12 notes
class 11 chemistry chapter 5 notes
some basic concepts of chemistry ncert solutions
heredity notes class 10
class 6 science notes chapter 1
ch 1 chemistry class 11 notes
class 10 political science chapter 1
class 10 chemistry chapter 3 notes
class 10 economics chapter 4 notes
plus one chemistry chapters
economics chapter 2 class 10 notes
chapter 4 physics class 12
class 9 science chapter 6 notes
hindi class 10 notes
circles class 10 notes
polynomial class 10 notes
trigonometry class 10 notes
ch 2 science class 10 notes
economics class 9 chapter 1 notes
organising class 12 notes
development class 10 economics notes
examfear notes
semiconductor class 12 notes
class 11 physics chapter 5 notes
class 11 physics notes chapter 1
class 10 chemical reactions and equations notes
surface chemistry class 12 ncert solutions
class 10 chapter 1 science notes
class 5 maths chapter 1 notes
accountancy class 11 chapter 1 notes
class 9 science chapter 13 notes
economics class 12 chapter 1
class 10 geography chapter 2 notes
chapter 3 physics class 12
some basic concepts of chemistry neet questions
the age of industrialisation class 10 notes
notes of history class 9 chapter 1
history class 10 chapter 1 notes in hindi
plus one physics chapters
class 12 history chapter 1 notes
class 10 geography chapter 7 notes
class 12 physics notes with derivations
notes of resources and development class 10
notes of economics class 10 chapter 2
ncert solutions for class 12 biology chapter 2
electromagnetic induction class 12 notes
class 10 science chapter 9 notes
9th class telugu 1st lesson notes
ch 1 civics class 10
respiration class 10 notes
class 10 history ch 1 notes
class 10 science electricity notes
class 12 chemistry chapter 3 notes
economics class 10 chapter 2 notes
chapter 2 economics class 10
class 12 biology chapter 1
geography class 10 chapter 4 notes
social science class 10 ncert solutions in hindi
chapter 1 biology class 11
class 11 chemistry chapter 1 solutions
class 10 science chapter 11 notes
class 11 biology chapter 3 notes
chapter 6 tissues class 9 notes
ncert solutions for class 11 biology chapter 2
class 11 bio chapter 1 notes
notes of political parties class 10
class 6 science chapter 6 notes
ch 1 chemistry class 12
some basic concepts of chemistry class 11 mcq with answers
democracy and diversity class 10 notes
bst notes class 12
physics wallah class 12 notes
globalization class 10 notes
class 12 geography ncert solutions
class 12 chemistry notes in hindi
the human eye and the colourful world notes
understanding quadrilaterals class 8 notes
class 9 science chapter 9 notes
bio notes class 12
electrochemistry class 12 handwritten notes
class 9th history chapter 1 notes
1st year physics notes
class 12 biology notes chapter 1
ch 12 science class 10 notes
class 10 acid base and salt notes
bio notes class 10
notes of manufacturing industries class 10
light and reflection class 10 notes
class 10 science chapter 15 notes
class 10 chemistry chapter 4 notes
chapter 3 chemistry class 11 notes
class 6 history chapter 1 notes
class 9 maths notes chapter 1
class 10 physics chapter 1 notes light reflection and refraction
consumer rights class 10 notes
science chapter 1 class 10 notes
geography class 9 chapter 1 notes
history class 9 chapter 1 notes
vector algebra class 12 notes
byju's notes class 10
class 12 business studies chapter 1
class 12 accountancy chapter 1 notes
chapter 3 physics class 11 notes
financial management class 12 notes
ncert notes for class 10 social science
class 11 maths chapter 1 sets notes
microbes in human welfare class 12 notes
class 12 biology chapter 5 notes
class 11 political science chapter 1
political parties class 10 ncert solutions
class 10 history chapter 1 notes in hindi
acid and base class 10 notes
chemistry class 10 chapter 2 notes
sports and nutrition class 12 notes
class 12 sociology notes
class 10 english chapter 1 notes
staffing class 12 notes
directing class 12 notes
economics chapter 1 class 9 notes
chapter 2 chemistry class 12 notes
class 8 social science notes chapter 1
class 10 manufacturing industries
determinants class 12 notes
class 10th science chapter 1 notes
class 11 biology chapter 4 notes
class 9 science chapter 10 notes
some basic concepts of chemistry notes class 11
class 11 biology chapter 1 the living world notes
ncert class 10 history chapter 1 notes
jee main chemistry some basic concepts in chemistry
economics chapter 1 class 11
surface area and volume class 10 notes
class 10 civics chapter 6 notes
chapter 2 physics class 12 notes
some basic concepts of chemistry notes pdf download
class 11 chapter 1 chemistry
class 10 political science chapter 1 notes
some applications of trigonometry class 10 notes
class 10 chapter 6 science notes
class 10 heredity and evolution
class 11 chemistry chapter 1 numericals pdf
ch 2 physics class 11 notes
psychology class 12 notes
class 10 biology chapter 3 notes
political science class 12 chapter 1 notes in hindi
thermodynamics class 11 physics notes
notes of chemical reaction and equation
class 11 chemistry chapter 1 exercise
physics class 12 chapter 1 in hindi
globalization and the indian economy class 10 notes
electric circuit class 10
chapter 6 class 10 science notes
class 10 chemistry chapter 5 notes
matter around us pure class 9 notes
class 11 chapter 1 chemistry notes
business environment class 12 notes
notes of economics class 10 chapter 1
class 10 science life process
notes of heredity and evolution class 10
electric charge and field class 12 notes
economics class 10 notes
class 12 political science chapter 1
ch 8 science class 10 notes
polymers class 12 notes
biology chapter 1 class 10
ch 10 science class 10 notes
chapter 1 chemistry class 12 notes
development chapter class 10 notes
sociology class 12 notes
physics class 12 chapter 2 notes
class 10 science chapter 1 notes in hindi
business studies class 12 chapter 1
notes of federalism class 10
physics class 9 chapter 1 notes
modern physics chapters class 12
class 9 chapter 1 science notes
physics wallah class 11 notes
notes of science class 10 chapter 1
principles of inheritance and variation class 12 notes
principles of management class 12 notes
biomolecules class 12 ncert solutions
ncert class 9 science notes in hindi medium









No comments:
Post a Comment